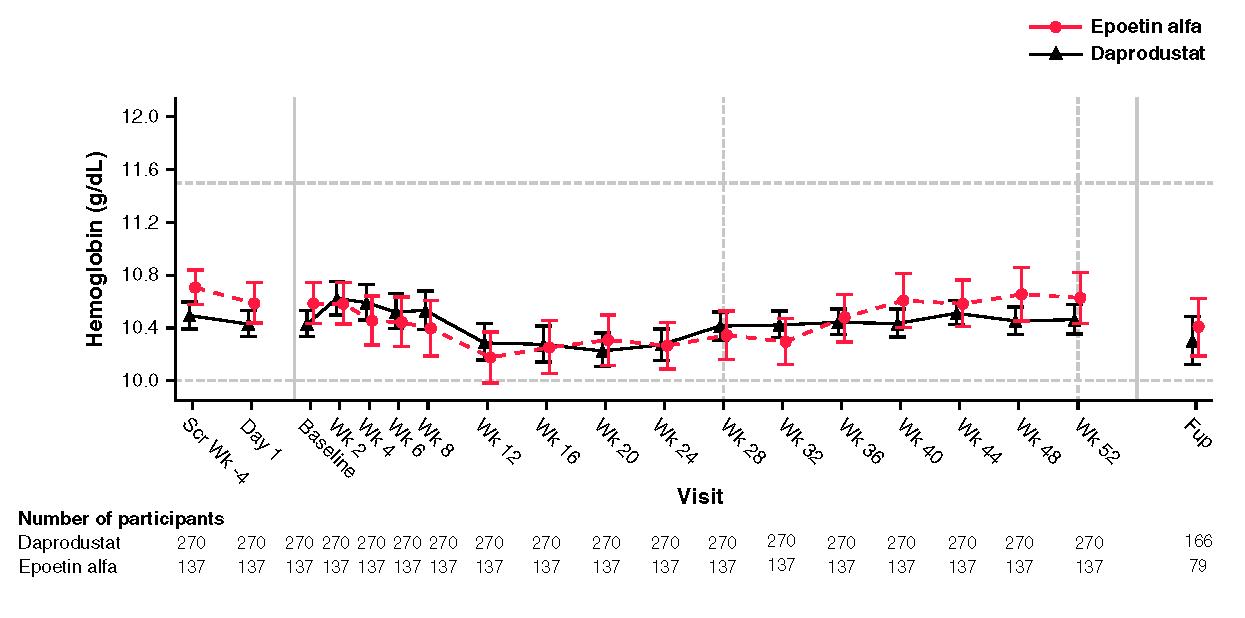
In early phase clinical trials Daprodustat* was administered as a once daily dose to hemodialysis patients. However, because standard clinical practice is to administer the competing drug, erythropoietin (“EPO”) three times weekly, it became essential to create a safe and effective thrice weekly dosing regimen for Daprodustat. This was accomplished in the ASCEND-TD clinical trial. Note that although this study used a non-inferiority design, FDA used the term “equivalent pharmacodynamic potency” to describe how the EPO and Daprodustat compare. So there is no statistical test for equivalence, although FDA provided that term in the approval package.
*FDA Approved February, 2023 for
JESDUVROQ is a hypoxia-inducible factor prolyl hydroxylase (HIF PH) inhibitor indicated for the treatment of anemia due to chronic kidney disease in adults who have been receiving dialysis for at least four months.
Limitations of Use Not shown to improve quality of life, fatigue, or patient well-being.
Not indicated for use: As a substitute for transfusion in patients requiring immediate correction of anemia. In patients not on dialysis.
Package insert Revised: 2020
https://doi.org/10.2215/CJN.00550122
@orphandrugservces
