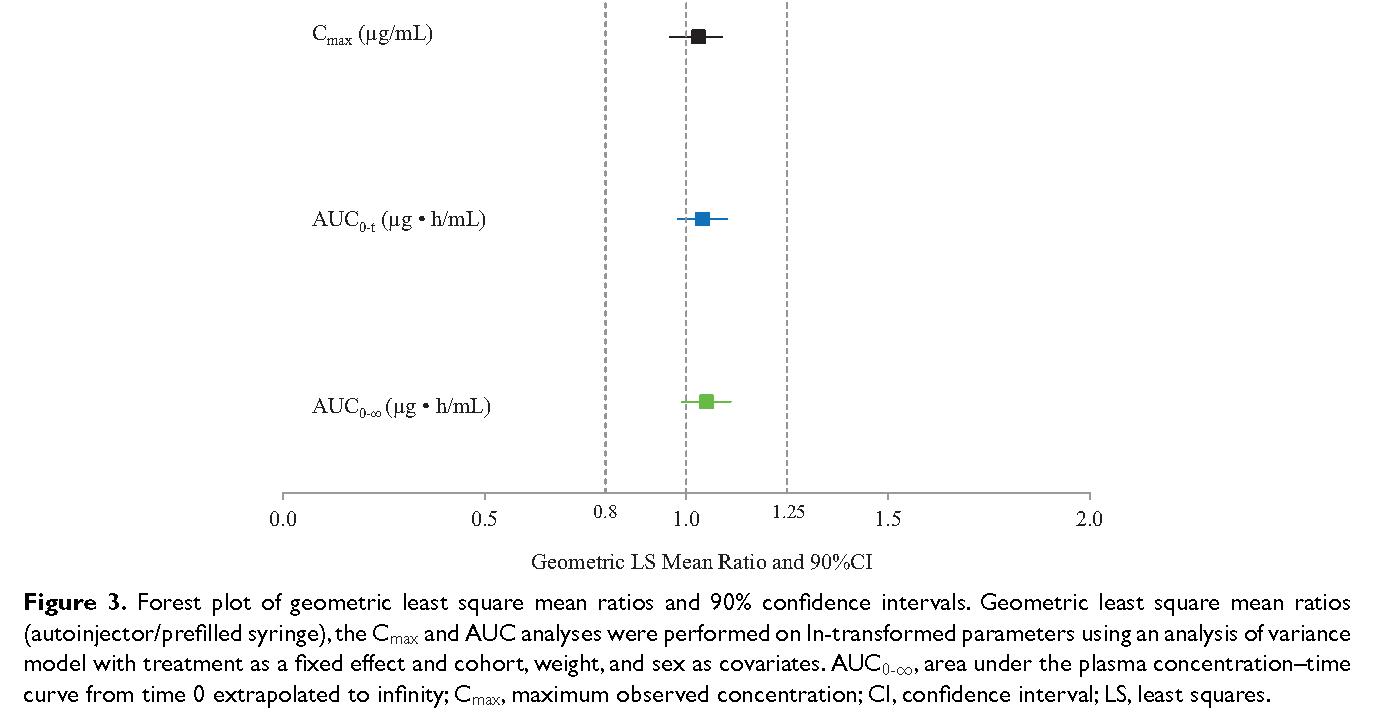
Fremanezumab* bioequivalence bridging studies were required to compare early formulations (vials, prefilled syringes) to marketed formulations (autoinjectors). Of note with a circulating half-life of approximately 29 days, blood sampling over 141 +/- 3 days was required, making for an exceptionally long and large (N=216) bioequivalence study. A parallel design was used. Despite those challenges, bioequivalence was clearly shown. Note that with fixed dose injectables, changes in drug concentration vs body weight and size need to be assessed.

*FDA Approved May, 2023 for AJOVY is a calcitonin gene-related peptide antagonist indicated for the preventive treatment of migraine in adults. (1)
Package insert Revised: 2020
DOI: 10.1002/cpdd.902
@orphandrugservces
